A remarkable chance discovery in a Cambridge University research lab shows that a number of life-sustaining metabolic processes can occur spontaneously and outside of living cells. This opens a rich, new vein of theories and approaches to studying the origin of life.
From the New Scientist:
Metabolic processes that underpin life on Earth have arisen spontaneously outside of cells. The serendipitous finding that metabolism – the cascade of reactions in all cells that provides them with the raw materials they need to survive – can happen in such simple conditions provides fresh insights into how the first life formed. It also suggests that the complex processes needed for life may have surprisingly humble origins.
“People have said that these pathways look so complex they couldn’t form by environmental chemistry alone,” says Markus Ralser at the University of Cambridge who supervised the research.
But his findings suggest that many of these reactions could have occurred spontaneously in Earth’s early oceans, catalysed by metal ions rather than the enzymes that drive them in cells today.
The origin of metabolism is a major gap in our understanding of the emergence of life. “If you look at many different organisms from around the world, this network of reactions always looks very similar, suggesting that it must have come into place very early on in evolution, but no one knew precisely when or how,” says Ralser.
Happy accident
One theory is that RNA was the first building block of life because it helps to produce the enzymes that could catalyse complex sequences of reactions. Another possibility is that metabolism came first; perhaps even generating the molecules needed to make RNA, and that cells later incorporated these processes – but there was little evidence to support this.
“This is the first experiment showing that it is possible to create metabolic networks in the absence of RNA,” Ralser says.
Remarkably, the discovery was an accident, stumbled on during routine quality control testing of the medium used to culture cells at Ralser’s laboratory. As a shortcut, one of his students decided to run unused media through a mass spectrometer, which spotted a signal for pyruvate – an end product of a metabolic pathway called glycolysis.
To test whether the same processes could have helped spark life on Earth, they approached colleagues in the Earth sciences department who had been working on reconstructing the chemistry of the Archean Ocean, which covered the planet almost 4 billion years ago. This was an oxygen-free world, predating photosynthesis, when the waters were rich in iron, as well as other metals and phosphate. All these substances could potentially facilitate chemical reactions like the ones seen in modern cells.
Metabolic backbone
Ralser’s team took early ocean solutions and added substances known to be starting points for modern metabolic pathways, before heating the samples to between 50 ?C and 70 ?C – the sort of temperatures you might have found near a hydrothermal vent – for 5 hours. Ralser then analysed the solutions to see what molecules were present.
“In the beginning we had hoped to find one reaction or two maybe, but the results were amazing,” says Ralser. “We could reconstruct two metabolic pathways almost entirely.”
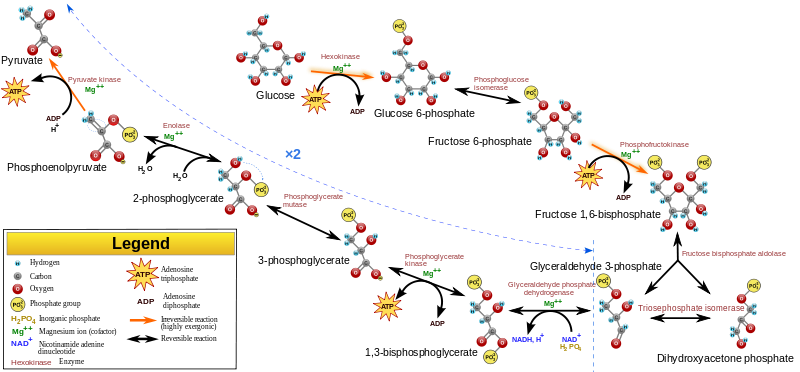
The pathways they detected were glycolysis and the pentose phosphate pathway, “reactions that form the core metabolic backbone of every living cell,” Ralser adds. Together these pathways produce some of the most important materials in modern cells, including ATP – the molecule cells use to drive their machinery, the sugars that form DNA and RNA, and the molecules needed to make fats and proteins.
If these metabolic pathways were occurring in the early oceans, then the first cells could have enveloped them as they developed membranes.
In all, 29 metabolism-like chemical reactions were spotted, seemingly catalysed by iron and other metals that would have been found in early ocean sediments. The metabolic pathways aren’t identical to modern ones; some of the chemicals made by intermediate steps weren’t detected. However, “if you compare them side by side it is the same structure and many of the same molecules are formed,” Ralser says. These pathways could have been refined and improved once enzymes evolved within cells.
Read the entire article here.
Image: Glycolysis metabolic pathway. Courtesy of Wikipedia.
 Send to Kindle
Send to Kindle